News
EXCITING NEWS AHEAD !!!!
Dr. Toledo is Interviewed on WNYT Regarding Telehealth
- Posted By: Practice Staff
- Category: Featured, Coronavirus Disease 2019 (COVID-19)
Dr. Aixa Toledo-Garcia spoke with NewsChannel13 about how TCFR is utilizing video chatting services to continue to provide care for our patients during this Covid-19 pandemic. Click "Read Full Article" for the WNYT link.
TCFR Donates to Camelot Print and Copy Center's 'Real Men Wear Pink Campaign.'
- Posted By: Practice Staff
- Category: Featured, Events
As many of you know, October is Breast Cancer Awareness month. Camelot Print and Copy Centers, the local printing company that we use here at TCFR, created a campaign to raise money that will go towards research for breast cancer. Our partners here at TCFR recently made a donation to sponsor Team Camelot’s Real Men Wear Pink Campaign. 1 out of every 8 women are diagnosed with breast cancer, and Team Camelot wanted the assistance of local businesses and offices to help them make a [...]
Dr. Toledo is Featured as a Co-Author in a Scientific Journal Article Regarding Behcet's Disease
- Posted By: Practice Staff
- Category: Featured, Medical Publications
Our Managing Partner, Dr. Aixa Toledo-Garcia, was published as a co-author in an August publication of an article regarding cardiovascular associates with Behcet's Disease. To access the link to this article, select the "Read Full Article" button below.
New Services Coming Soon to TCFR!
- Posted By: Practice Staff
- Category: Featured, Events
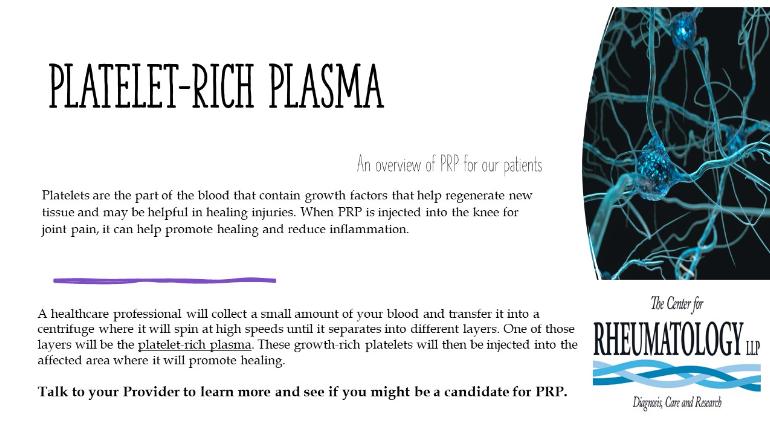
TCFR is happy to announce that Dr. Aixa Toledo-Garcia has recently been trained and obtained her certification in Regenerative Medicine. Dr. Toledo will soon be providing Prolotherapy and PRP (Platelet-Rich Plasma) therapy.